French to English Medical Translation: 5-Step Flawless Guide
Why Accurate French to English Medical Translation Matters
French to English medical translation is essential for patient safety, regulatory compliance, and effective global healthcare communication. For clinical trial documents, patient records, or medical device instructions, accuracy isn’t optional–it’s critical.
Here’s what you need to know about French to English medical translation:
- Professional translators: Require subject matter expertise, bilingual fluency, and deep knowledge of medical terminology.
- Certification requirements: Official translations need a Certificate of Accuracy and compliance with ISO 17100:2015 standards.
- Common documents: Clinical trial protocols, Informed Consent Forms (ICFs), Instructions for Use (IFUs), patient records, and pharmacovigilance reports.
- Pricing: Ranges from $0.09 to $0.25 per source word, based on language pair and document complexity.
- Quality assurance: Involves dual review (the “four-eye principle”), translation memories, and compliance with EMA, FDA, and ISO 13485.
- Technology’s role: AI and CAT tools speed up translation, but human medical experts are vital for final review and validation.
The stakes are high. A single mistranslation can lead to misdiagnosis, incorrect treatment, or legal issues. This field demands more than linguistic fluency–it requires an understanding of healthcare systems, regulatory frameworks, and the nuanced terminology that differs between French-speaking regions (France, Canada, Belgium).
I’m William Kennedy, a Translation Project Manager who has overseen more than 8,500 language projects, including extensive French to English medical translation work for pharmaceutical companies, clinical research organizations, and medical device manufacturers. My experience covers complex multilingual projects requiring meticulous attention to regulatory compliance, terminological consistency, and quality assurance.
Relevant articles related to french to english medical translation:
The Unique Challenges and Requirements of Medical Translation
In healthcare, language barriers aren’t just inconvenient–they can be dangerous. Translating medical documents from French to English involves navigating complex medical terminology, cultural nuances, and strict regulatory requirements. The precision required is absolute because even a small error can have serious, sometimes life-threatening, consequences.
Primary Challenges in Translating French Medical Documents
French to English medical translation presents unique problems that demand specialized expertise. Here are the most common challenges:
False friends (faux amis) are words that look similar but have different meanings. For example, the French word “actuellement” means “currently,” not “actually.” In a medical context, this confusion can lead to serious misinterpretations of a patient’s condition.
Acronyms and abbreviations add complexity. While some are international, many are specific to French-speaking healthcare systems. A translator must know that “AVC” (accident vasculaire cérébral) translates to “stroke” or “CVA” (cerebrovascular accident) in English.
Eponyms–terms named after people, like Alzheimer’s disease–can also be a challenge. Some French medical eponyms lack direct English equivalents and require careful research or explanation.
Different healthcare systems in France, Canada, and Belgium have distinct legal requirements and medical practices. A skilled translator must understand these systemic differences to accurately convey information.
The implications of inaccurate medical translations are severe. A mistranslated dosage, diagnosis, or device instruction can jeopardize patient safety, leading to legal liabilities and regulatory violations. Meticulous accuracy is non-negotiable.
Linguistic variants are a final consideration. Canadian French, for example, has unique terminology and Anglicisms that differ from European French. Your translator must be familiar with the specific regional variant of the source document to capture its precise meaning.
Common French Medical Documents for Translation
The demand for French to English medical translation covers a wide range of documents, including:
- Clinical trial documents: Protocols, investigator brochures, case report forms (CRFs), and clinical study reports vital for global drug development and regulatory submissions to bodies like the FDA and EMA.
- Patient records: Medical histories, discharge summaries, lab results, and imaging reports for patients seeking care abroad or for insurance and legal purposes.
- Informed Consent Forms (ICFs): Legally and ethically critical documents that ensure patients understand the risks and benefits of a procedure or clinical trial.
- Instructions for Use (IFUs): User manuals for medical devices where clarity is essential to prevent misuse and patient harm.
- Pharmacovigilance reports: Documents that track adverse drug reactions as part of international safety monitoring.
- Scientific articles and research papers: Translated for publication in international journals to contribute to global medical knowledge.
- Regulatory documents: Certified translations for submissions to agencies like the EMA or FDA, including medical patents.
- Public health information: Materials on conditions like asthma, cancer, and diabetes that need to be accessible to diverse communities.
Key Qualifications for a French to English Medical Translator
Not just anyone who speaks French and English can translate medical documents. The role requires exceptional expertise.
- Subject matter expertise: Our translators are not just linguists; many are medical professionals like doctors or researchers with deep knowledge of medicine and biotechnology.
- Bilingual fluency: Translators must have a native-level command of both French and English, including cultural nuances and idioms.
- Understanding of medical terminology: This requires profound knowledge of anatomical terms, diseases, procedures, and pharmacology in both languages, including standardized terminologies like Snomed-CT and ICD-10.
- Knowledge of regulations: Our translators understand the requirements of the FDA, EMA, and ISO 13485, ensuring translations meet all necessary standards.
- Years of experience: Our expert linguists often have over 20 years of experience, demonstrating a proven track record of accuracy.
- Professional association membership: Many of our translators are members of organizations like the American Translators Association (ATA) and hold specific certifications in medical translation, validating their expertise.
Ensuring Accuracy and Compliance in French to English Medical Translation
Accuracy and compliance aren’t just buzzwords in French to English medical translation–they’re essential safeguards that protect patient safety and ensure your documents meet regulatory requirements. Every word matters when health outcomes are on the line.

At Latitude Prime, our quality assurance process uses multiple layers of review, careful terminology management, and strict adherence to international regulations. We don’t cut corners, because we know you can’t afford to either.
How Services Ensure Accuracy and Regulatory Compliance
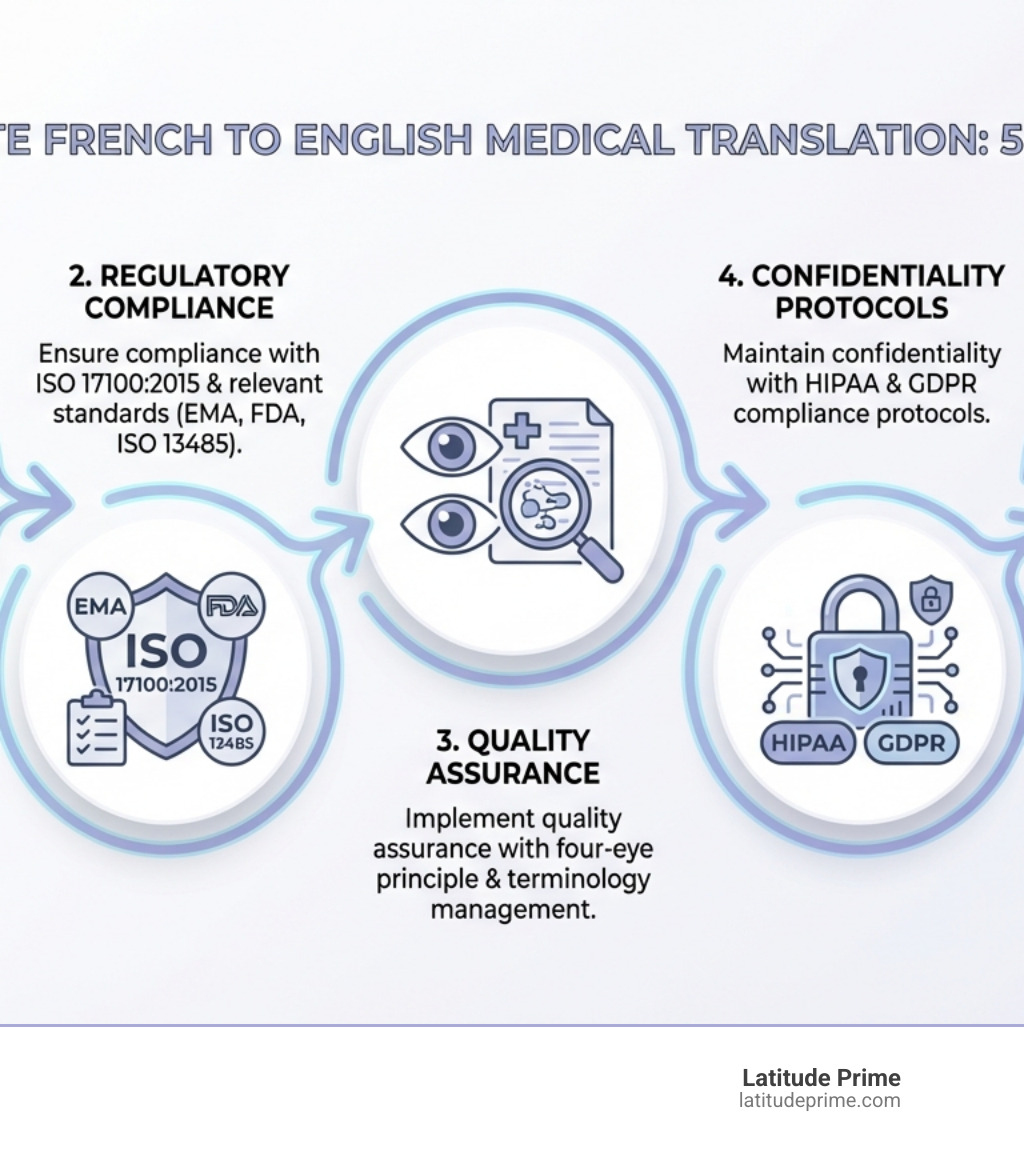
Delivering accurate and compliant translations requires a systematic approach. We start with international quality standards like ISO 17100:2015, and our quality management system is audited and certified to meet this standard. This gives you confidence that every project follows proven best practices.
We also ensure compliance with EMA, FDA, and ISO 13485 standards for pharmaceutical and medical device documentation. These are mandatory requirements for regulatory submissions and market access.
The four-eye principle is the backbone of our quality control. After the initial translation, a second independent medical translator reviews the entire document. This dual review catches errors, inconsistencies, and nuances that a single translator might miss.
For highly sensitive documents, we use back translation to verify accuracy. An independent translator, who has not seen the original French text, translates the English version back into French. This process reveals any ambiguities that may have entered the English translation.
When linguistic accuracy isn’t enough–you need in-country review. We facilitate reviews by English-speaking medical professionals who verify that the translation uses terminology that local practitioners will immediately understand.
Throughout this process, we maintain strict compliance with HIPAA and GDPR regulations, protecting all patient data. Your protected health information (PHI) remains confidential and secure.
The Role of Certification and Confidentiality
Medical translations often need more than just accuracy–they need official validation. That’s where certification comes in.
Certified translations are required for regulatory submissions (FDA, EMA), legal proceedings, and immigration applications. Without certification, a perfect translation may not be accepted.
We provide a Certificate of Accuracy with every medical translation. This signed statement on our company letterhead attests that the translation is accurate and complete, providing proof that the work was done professionally. It is often accompanied by our ISO 17100:2015 quality certificate.
Confidentiality is equally critical. Our platforms are SOC 2 Type II compliant and feature end-to-end encryption, strict access controls, and full HIPAA and GDPR compliance. We are happy to sign a confidentiality agreement (NDA), and all our staff are bound by strict confidentiality agreements.
Maintaining Terminological Consistency
Medical terminology must be consistent across all documents to avoid confusion and maintain credibility.
We use Translation Memory (TM) tools that store previously translated segments. When new documents contain matching text, the TM suggests the previous translation, ensuring consistency and speeding up the process, especially for updates.
For each client, we develop comprehensive glossaries and termbases–specialized databases of medical terms. These ensure that key terms, acronyms, and concepts are translated the same way every time.
We also create client-specific style guides that outline preferences for tone, formatting, and linguistic conventions. This ensures your translated content aligns with your brand voice.
The combination of TM, glossaries, and style guides allows us to maintain unparalleled consistency across long-term projects, which is crucial for multi-year clinical trials or comprehensive product documentation.
Human vs. Technology: Choosing the Right Translation Method
A common question in French to English medical translation is whether AI can handle the job. It’s a fair question, given the advances in machine translation. But the truth is–in medical translation, it’s not about choosing between human expertise or technology. It’s about knowing how to combine them effectively.
Let me break down what we’ve learned from managing thousands of medical translation projects.
| Feature | Human Translation | Technology-Assisted Translation (AI/MT) |
|---|---|---|
| Nuance & Context | Excellent, understands subtleties, cultural implications. | Limited, struggles with ambiguity, idiomatic expressions. |
| Speed & Volume | Slower, limited by human capacity. | Very fast, handles large volumes quickly. |
| Cost-Effectiveness | Higher per-word cost. | Lower per-word cost, especially for repetitive content. |
| Quality & Accuracy | Highest, especially with subject matter expertise & review. | Varies, requires significant post-editing by human experts for medical. |
| Critical Thinking | Applies medical knowledge, ethical judgment. | Lacks critical judgment, cannot assess medical validity. |
| Confidentiality | Relies on translator ethics & NDAs. | Depends on platform security, HIPAA/GDPR compliance. |
The Case for Professional Human Translators
There’s a reason we insist on human experts for medical documents–and it goes far beyond just getting the words right.
Understanding context is where human translators shine. They interpret meaning, understand implications, and recognize when something doesn’t add up. A human translator with medical expertise can spot when a dosage instruction needs clarification because the source text is ambiguous.
Cultural adaptation is also key. Healthcare practices differ globally. A professional translator understands these differences and can bridge the gap naturally for the target audience.
Then there’s handling ambiguity. Medical texts can be unclear or contain rushed notes. A human translator with clinical knowledge will flag these passages for clarification rather than guess.
Critical thinking and medical judgment are perhaps the most crucial elements. Our translators, many of whom are doctors or researchers, apply their medical knowledge to ensure the translation makes clinical sense.
Final review and editing by a human expert is non-negotiable for us. This is where we catch subtle errors, ensure consistency, and verify that all regulatory requirements are met.
When human expertise is essential: If a mistranslation could harm a patient, you need a human expert. Period. Drug labels, surgical instructions, and clinical trial protocols–these aren’t documents where “good enough” is acceptable.
The Role of Technology in Modern Translation
While we’re adamant about human oversight, we’re equally enthusiastic about technology–when used correctly.
Technology-assisted platforms, like computer-assisted translation (CAT) tools, have transformed our workflow. They help us coordinate projects, track progress in real-time, and ensure quality.
Increased efficiency is a major contribution. Translation Memory (TM) systems store previously translated segments, so we don’t start from scratch on updates. This improves consistency and can cut translation time from weeks to days.
Our terminology management systems work similarly, ensuring that key terms are translated consistently across all your documents.
Technology with human review is our sweet spot. We may use AI to create first drafts for standardized content, but–and this is crucial–those drafts always go to specialized human translators for thorough review and editing. This hybrid approach combines the speed of AI with the validated accuracy of medical experts.
High-quality results with expert oversight come from this combination. Technology handles repetitive tasks and maintains consistency, while human experts apply their medical knowledge, cultural understanding, and critical judgment.
Text extraction from scanned PDFs is another game-changing feature. Our AI Agents use Optical Character Recognition (OCR) to extract text from any medical document, even handwritten notes, while preserving the original layout and saving hours of reformatting work.
The bottom line? For French to English medical translation, technology is a powerful tool. But the expertise, judgment, and accountability still come from our human translators. That’s the combination that delivers fast, accurate, and secure medical translations.
How to Find and Choose a Reliable Translation Provider
Selecting a reliable provider for your French to English medical translation is a critical decision. The accuracy and compliance of your medical documents depend on the expertise and processes of your chosen partner.
This section will guide you through the vetting process, helping you ask the right questions and evaluate potential translation services.
Steps to Find a Reliable Provider
When entrusting your critical medical documents for translation, follow these steps to ensure you partner with a trustworthy service:
- Check for Certifications (ISO or Equivalent): Look for providers certified to international standards like ISO 17100:2015. This demonstrates a commitment to quality processes. Also, ensure they comply with medical industry standards such as ISO 13485, EMA, and FDA regulations.
- Review Case Studies and Testimonials: A reputable provider will have a portfolio of successful projects and client testimonials. Look for examples of their work with organizations similar to yours to see real-world evidence of their capabilities.
- Ask for Linguist Qualifications: Inquire about the qualifications of their French to English medical translators. Are they native English speakers with subject matter expertise (e.g., medical degrees)? Are they certified by professional bodies like the American Translators Association (ATA)?
- Assess Their Quality Management System: Understand their internal quality assurance processes. Do they use the “four-eye principle” (translation and review by separate experts)? Do they use translation memories and glossaries for consistency? A robust system indicates a commitment to high-quality results.
- Choose Specialized Services: Opt for specialized medical translation services over general agencies. Specialists have dedicated medical linguists, industry knowledge, and established workflows for handling sensitive data and adhering to strict regulatory requirements.
Understanding the Costs of Medical Translation
The cost of French to English medical translation varies, and it’s important to understand the influencing factors. While cost is a consideration, it should never be the sole determinant for medical documents.
- Pricing Models (Per Word): Most professional services charge per source word, which provides transparency and allows for easy cost estimation.
- Factors Affecting Cost:
- Language Pair: The specific expertise required for French to English medical content can influence the rate.
- Document Complexity: Highly technical documents with specialized terminology generally cost more than simpler patient information leaflets.
- Turnaround Time: Rush projects requiring expedited delivery will typically incur higher rates.
- Volume: Larger projects may qualify for volume discounts.
- Value-Added Services: Certification, notarization, back translation, or desktop publishing (DTP) will add to the overall cost.
- Cost Range: Industry averages for medical translation range from $0.09 to $0.25 per source word, depending on the language pair and complexity.
Common Pitfalls in French to English Medical Translation
Avoiding common pitfalls can save time, money, and prevent serious consequences in French to English medical translation.
- Choosing Based on Price Alone: Selecting a provider solely on the lowest price is a risky gamble. Cheap services often cut corners on quality assurance or use unqualified translators, leading to inaccurate results.
- Using Generalist Translators: A translator fluent in general French may not possess the specialized medical knowledge required, leading to misinterpretation of jargon, symptoms, or drug names.
- Ignoring Linguistic Variants: French has regional variants (France, Canada, Belgium). Using a translator unfamiliar with the specific variant can lead to critical misinterpretations of terminology.
- Over-Relying on Raw Machine Translation: Using unedited machine output for medical documents is extremely dangerous. Tools like Google Translate lack the human judgment and cultural understanding necessary for medical contexts.
- Lack of a Quality Review Process: A provider that doesn’t detail a robust quality assurance process (like the four-eye principle or terminology management) is a red flag. Skipping these steps significantly increases the risk of errors.
Frequently Asked Questions about French Medical Translation
When you’re dealing with French to English medical translation, you likely have questions. These are the ones we hear most often, and we want to give you straightforward answers.
What is the difference between a certified translation and a standard translation?
A standard translation accurately converts your French medical document into English for informational purposes. A certified translation adds a formal “Certificate of Accuracy”–a signed statement from the translation provider attesting to the translation’s accuracy and completeness.
This certification is crucial because many official bodies require it. If you’re submitting medical documents to USCIS for immigration, the ECFMG for medical licensing, academic institutions, or regulatory bodies like the FDA or EMA, they will likely ask for a certified translation as assurance that it was professionally done.
We provide a certificate of accuracy with every medical translation we complete, so you’re always prepared for official requirements.
How long does a medical translation project typically take?
The timeline depends on several factors: document length, complexity, and formatting. A one-page patient summary will be faster than a 200-page clinical trial protocol. Complex layouts or additional services like back translation also extend the timeline.
However, our technology and expert teams dramatically speed up this process. We use translation memories and efficient workflows to deliver projects that once took weeks in just a few days. A typical medical record might be ready within 24 hours, while a comprehensive report could take several days to two weeks, depending on its scope. For rush projects, we can often deliver 10,000 words or more per day.
When you contact us, we’ll provide a realistic timeline based on your exact needs.
Can you translate a handwritten doctor’s note from French?
Yes, we can–and we do it regularly. Handwritten medical notes are a common challenge, but our expert medical translators can work with them, provided the handwriting is reasonably legible.
Our linguists have years of experience reading medical handwriting in both French and English, so they are familiar with common abbreviations and medical shorthand. We also use technology to help. Our tools with Optical Character Recognition (OCR) can extract text from scanned handwritten documents, which our translators then review and correct against the original image.
Our process is thorough: after the initial translation, a second medical expert reviews it using our four-eye principle. If we encounter passages we can’t confidently decipher, we’ll flag them for you and, if possible, suggest reaching out to the original provider for clarification.
Conclusion
When it comes to healthcare communication across borders, getting the words right isn’t just important–it’s everything. We’ve walked through the intricate world of French to English medical translation, from subtle linguistic traps to the rigorous quality standards that protect patient safety.
Medical translation demands more than fluency. It requires specialized expertise, an understanding of complex healthcare systems, and an unwavering commitment to accuracy. A misplaced decimal point in a dosage or a misunderstood term in a consent form–these aren’t just errors. They are potential threats to patient wellbeing and regulatory compliance.
That’s why partnering with a certified provider makes all the difference. Professional human translators with medical backgrounds, combined with smart technology, create the gold standard in medical translation. This approach ensures your documents meet the exacting standards of bodies like the FDA and EMA while maintaining the consistency and confidentiality your organization requires.
At Latitude Prime, we understand what’s at stake. Our ISO 17100:2015 certified quality management system, network of expert medical linguists, and commitment to HIPAA and GDPR compliance mean your most sensitive medical documents are in capable hands. Whether you’re translating a single patient record or managing a complex multilingual clinical trial, we bring the precision and security your project demands.
Ready to ensure your medical translations meet the highest standards? Get a quote for your French medical translation project and experience the difference that specialized expertise makes.


